

 External Ventricular Drainage Device
External Ventricular Drainage Device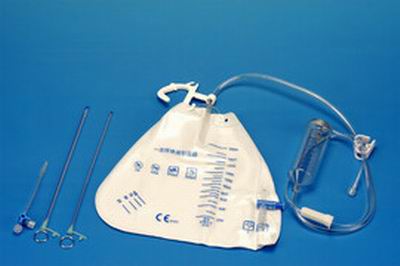
Application Scope:
lDrainage for intracranial hemorrhage
lDrainage for intracranial infection
lDrainage after intracranial tumor surgery
lCatheter intubation before some surgery to reduce
intracranial pressure
lAcute hydrocephalus caused by brainstem hemorrhage or
cerebellar hemorrhage entering encephalocoele
lDrainage for Subdural hematoma or epidural hematoma
Type Ⅰ
|
Code |
Specification |
|
5201 |
9Fr, simple package |
|
5202 |
9Fr, composite package |
Components:
lDrainage catheter with vent
lConnection tubing
lFlow regulator
lDrainage chamber
lCollection bag
lTubing clamp
lCatheter clamp
lRuler
Characteristic:
lCatheter is made of PU with high rigidness, thin
wall, high flow, smooth, good biocompatibility,
blind end, less harm, radiopaque line.
lCatheter is with vent to supply perfect test platform.
Drainage and continuous intracranial pressure test
(or medicine supply) can be performed simultaneously.
lDrainage chamber and collection bag is with tick mark
to measure the volume of liquid conveniently. It is
equipped with gas filter.
lThe collection bag is with one way valve to avoid
infection.
Type Ⅱ
|
Code |
Specification |
|
5301 |
10Fr, simple package |
|
5302 |
10Fr, composite package |
|
5303 |
14Fr, simple package |
|
5304 |
14Fr, composite package |
Components:
lDrainage catheter
lExtension line with stopcock
lConnection tubing
lFlow regulator
lDrainage chamber
lCollection bag
lTubing clamp
Characteristic:
lCatheter is made of medical silicone with blue
radiopaque line, blind end.
lDrainage chamber is equipped with gas filter
to avoid contra-flow and syphonage.
lDrainage chamber and collection bag is with tick mark
to measure the volume of liquid conveniently. It is
equipped with gas filter.
lThe collection bag is with one way valve to avoid
infection.
lHypodermic needle and cranial awl can be equipped
if needed.